An Update on SKY-0515
January 26, 2026

HDYO has more information about HD available for young people, parents and professionals on our site:
www.hdyo.org
Skyhawk Therapeutics released an update on their SKY-0515 study earlier today. Below is their community letter and press release available for translations.
Dear friends in the Huntington’s disease community,
We’d like to share an update on ongoing research into SKY-0515, an investigational medicine being studied for Huntington’s disease (HD). A scientific press release was shared today, and this letter is meant to explain the news in a more community-focused, easier-to-read way.We know how important it is for families and individuals affected by HD to receive clear, honest updates, and we appreciate you taking the time to read this.
What is SKY-0515?
SKY-0515 is an investigational, once-daily oral medicine that is currently only available through clinical trials. It is not approved for use in any disease or in any country. The investigational medicine is designed to affect two biological factors involved in Huntington’s disease:
Mutant huntingtin (mHTT), the abnormal protein that causes HD
PMS1, a protein that may be involved in processes linked to disease progression
Researchers are studying whether changes in these biological markers could be relevant to HD over time.
What was shared in the January 27th announcement?
The announcement included results from a nine-month interim analysis of people with early-stage HD who are participating in a Phase 1 clinical study. This study is focused mainly on safety and on understanding how the investigational medicine behaves in the body. Here is what was observed so far:
Changes in biological markers
People taking SKY-0515 showed dose-dependent reductions in mutant huntingtin levels in blood
At the higher dose studied, average reductions of about 62% were seen
Reductions in PMS1 mRNA of about 26% were also observed
The investigational medicine was shown to reach the brain, which is essential for treating HD
These findings suggest that SKY-0515 is interacting with its intended biological targets. At this stage, it is not known how these changes relate to symptoms or long-term outcomes.
What do we know about safety?
SKY-0515 has been generally well tolerated in the study so far
No significant safety concerns were identified in the data reported
Safety continues to be closely monitored as the studies move forward
Were symptoms or function measured?
Researchers also collected exploratory clinical assessments, including a combined score called the Composite Unified Huntington’s Disease Rating Scale (cUHDRS), which looks at movement, thinking, and daily function.
Average scores were generally stable over time, with small changes observed
After nine months, participants on SKY-0515 showed small average improvements from baseline
Because this is an early-phase study with a limited number of participants, these findings should be interpreted with caution
Larger and longer studies are needed to better understand whether SKY-0515 may have an effect on symptoms or disease progression.
What studies are happening now?
Phase 1 study
Enrollment is complete
Participants are continuing in a blinded extension period
Additional data are expected in mid-2026
Phase 2/3 FALCON-HD study
Currently enrolling in Australia, New Zealand and Georgia. Other countries will be added soon
Participants will receive treatment or placebo for at least 12-18 months
This study will further evaluate safety, biological markers, and clinical measures
More details are available at ClinicalTrials.gov NCT06873334 and www.FALCON-HD.com.
What does this update mean for the HD community?
These results represent early but meaningful progress in the clinical development of SKY-0515. The findings show that the medicine is having measurable biological effects, but it is still too early to know what this may mean for people affected with HD. SKY-0515 remains an investigational treatment, and further research is needed before any conclusions can be drawn about its potential role in HD care. We remain committed to sharing updates transparently as the science evolves.
Stay Engaged Over the coming months, members of the Skyhawk team will participate in community events, conferences, and webinars hosted by several patient advocacy organizations, including Huntington’s Australia, Huntington’s Victoria, Huntington’s Disease Association of Auckland, Huntington’s Disease Association of America, Huntington Society of Canada, Huntington’s Disease Youth Organization, Factor-H, Association Huntington France and Huntington’s Disease Association of England and Whales. These organizations will share details about dates, times, and how to join through their usual channels, so we encourage you to follow them for the latest updates.
We are deeply thankful to the individuals and families who take part in research studies, and to the advocacy organizations and community members who continue to support HD research. We know the community has been waiting a long time for progress, and we are grateful that you continue to engage with us despite the uncertainties. Thank you for placing your hope and trust in research.
We remain committed to sharing updates as the research progresses.
With appreciation, The Skyhawk Therapeutics Team
----PRESS RELEASE-----
Skyhawk Therapeutics Announces Nine Month Interim Results in Patients from its Phase 1 Clinical Trial of SKY-0515 as a Treatment for Huntington’s Disease
Nine-month findings show mean improvement in Composite Unified Huntington's Disease Rating Scale from baseline of +0.64 points, compared to natural history expected worsening of cUHDRS in symptomatic patients of -0.73 points over nine months, based on propensity score weighting. Skyhawk also announces SKY-0515’s Phase 2/3 FALCON-HD trial has expanded worldwide. Skyhawk has now dosed more than 90 patients.
BOSTON, MA., January 27, 2026 - Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies to modulate critical RNA targets, today announces positive results from the nine month interim analysis of the Company’s investigational treatment for Huntington’s disease (HD) with SKY-0515.
Treatment with SKY-0515 results in dose-dependent reductions of mHTT protein in blood of 62% at the 9mg dose, and dose-dependent PMS1 mRNA reduction of 26%. PMS1 is a key driver of somatic CAG repeat expansion and HD pathology. SKY-0515 has also demonstrated excellent central nervous system exposure and been generally safe and well tolerated.
At three, six and nine months, patients receiving SKY-0515 in the Part C patient cohort of the Phase 1 clinical trial of SKY-0515, demonstrate mean Composite Unified Huntington's Disease Rating Scale (cUHDRS) improvement from baseline. At nine months, in a pooled analysis, this improvement is +0.64 points compared to expected worsening at nine months of cUHDRS in symptomatic patients of -0.73 points, based on propensity score weighting using Enroll-HD and TRACK-HD.
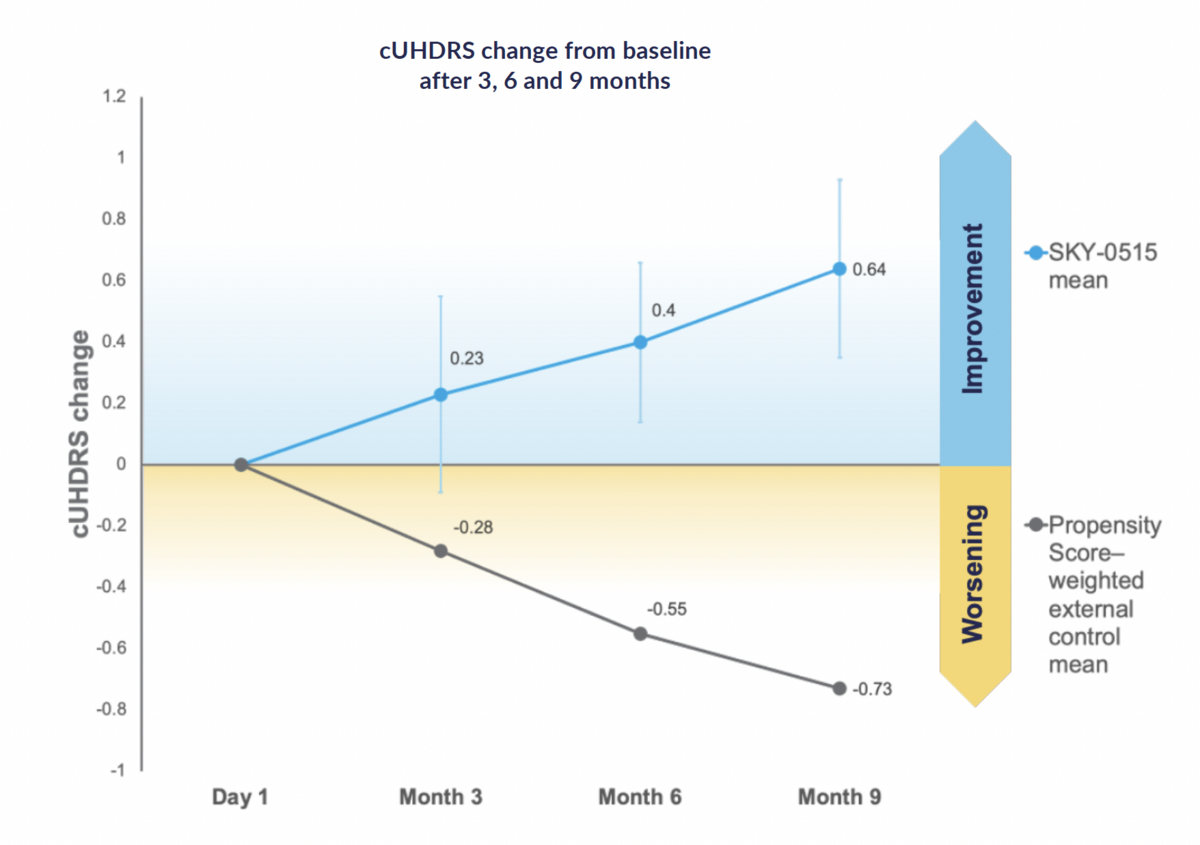
Skyhawk Therapeutics, Inc. 'Phase 1 Part C patient cohort cUHDRS and its components at 3, 6 and 9 months for patients who received SKY-0515 continuously for 9 months, once a day, with the data at 4mg and 9mg pooled (n=17),' January 2026. Note: Error bars represent standard error of the mean; Propensity score weighting (n=325) was performed using Enroll-HD and TRACK-HD.
“I am very encouraged by these safety and early efficacy data from SKY-0515’s Phase 1 Part C trial in patients, showing divergence in cUHDRS away from expected natural history deterioration at the three, six, and nine month prespecified analyses,” said Ed Wild, Professor of Neurology at University College London. “SKY-0515 continues to reduce mHTT protein to the greatest extent demonstrated by any therapeutic tested to date in patients, with clinical and biomarker data showing the drug is well tolerated at all doses tested. SKY-0515’s ability to reduce both mHTT and PMS1 offers a potent combination for treating Huntington’s disease via two of its core pathogenic mechanisms. These open-label trial results, due to be validated in the ongoing placebo-controlled FALCON-HD trial, give an expectation of meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 will be truly transformative."
“Our goal for our Phase 1 study was to establish safety and biomarker activity,” said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics, “and the continued strength of SKY-0515’s biomarker response in our nine month interim data analysis – and the improvement in the potential endpoint, cUHDRS, compared to a worsening of the cUHDRS score in the natural history data for patients - underscores SKY-0515’s potential as a best in class disease-modifying therapy for HD. These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk’s platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies.”
Huntington’s disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is an orally-administered, investigational small molecule RNA modulator developed through the company's novel RNA-modulating platform, SKYSTAR®. SKY-0515 therapeutically reduces both HTT protein and PMS1 protein. PMS1 is an additional key driver of somatic CAG repeat expansion and HD pathology and should complement the benefits of reducing mutant HTT.
Skyhawk also announces today that its SKY-0515 Phase 2/3 FALCON-HD trial, open at twelve sites in Australia and New Zealand, has expanded worldwide. Skyhawk has now dosed more than 90 patients with SKY-0515. SKY-0515 is the first Skyhawk drug in clinical trials.
Skyhawk expects to put additional small molecule drugs to treat rare neurological diseases with no approved disease modifying therapies in the clinic by the end of 2027.
About SKY-0515’s Phase 1 Clinical Study
SKY-0515’s Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SKY-0515 in healthy volunteers and individuals with early-stage Huntington’s disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in Healthy Volunteers. Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo in individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) for 84 days followed by a 12 month extension of active treatment where all participants will receive either a low or high dose of SKY-0515 in a blinded fashion. The objectives of the study include evaluating mutant HTT protein and PMS1 mRNA. The first patients were dosed in SKY-0515’s Part C in January 2025. Enrollment in Phase 1C of the SKY-0515 trial is now complete.
About SKY-0515’s Phase 2/3 FALCON-HD Clinical Study
FALCON-HD (NCT06873334) is a Phase 2/3 randomized, double-blind, placebo-controlled, dose ranging study to evaluate the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 participants with Stage 2 and early Stage 3 HD across 12 sites in Australia and New Zealand, and 400 participants with Stage 2 and early Stage 3 HD in 40+ worldwide sites. Eligible patients will receive a once-daily oral dose of SKY-0515 at one of three dose levels or placebo, for a treatment period of at least 12 months. The trial aims to assess the potential of SKY-0515 to modulate RNA splicing and reduce mHTT and PMS1 proteins, which are implicated in the pathology of Huntington's disease. Additional information about FALCON-HD, including participating sites and eligibility criteria, can be found at ClinicalTrials.gov and www.FALCON-HD.com.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company which uses its proprietary platform, SKYSTAR®, to discover and develop small molecule RNA modulating therapies for the world's most intractable diseases. For more information visit www.skyhawktx.com.
Skyhawk Contact Maura McCarthy Head of Corporate Development maura@skyhawktx.com
